Nitroreductase-based clostridium Directed Enzyme Prodrug Therapy (CDEPT) has shown promise in preclinical investigations as a cancer gene therapy treatment strategy, but it has yet to enter human clinical trials. This is likely owing to a number of factors, including concerns about human safety, the inability to monitor non-invasively the geographical and temporal distribution of C. sporogenes replication, and regulatory restrictions on endospore preparation for clinical application.
A method for chromosomal integration of heterologous genes into C. sporogenes was recently disclosed, allowing for the development of clinical grade endospore preparations that meet clinical regulatory standards. Neisseria meningitidis type I nitroreductase (NmeNTR) was one of the first enzymes to be incorporated in this way, and the research team wanted to pair it with the clinical-stage prodrug PR-104.
PR-104 was originally designed as a hypoxia-activated cytotoxin, but it has now proven to be a great bacterial nitroreductase substrate with a lot of bystander action. The team successfully overexpressed NmeNTR in an E. coli SOS reporter system as well as a human cell line, and found that PR-104A had higher activity than CB1954. In an experimental model of CDEPT, this increase in activity in vitro translated to significant in vivo efficacy for this enzyme/prodrug combination, but only in the context of significant tumour necrosis.
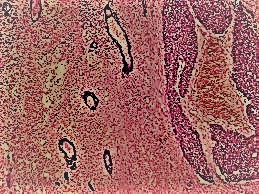
In spore-treated groups with minimally necrotic xenografts (5%), there was no in vivo anti-tumour activity. The vascular disrupting drug vadimezan enhanced tumour colonisation, resulting in considerable anti-tumour activity in all spore-treated groups (TGD67%) compared to untreated control animals. These finding highlights the remarkable selectivity of C. sporogenes germination for necrotic tumours, which is critical for safety and a distinguishing feature of this gene delivery platform over others. This Research was publish in Cancer Gene Therapy.
The vascular disrupting drug was given 60 minutes after the spores to prevent interfering with spore distribution to the tumour microenvironment. Following the delivery of spores, vascular collapse could theoretically lower the oxygen tension around the already imprisoned spores, increasing the chance of bacterial germination. Only the mice given vadimezan + spores had a RECIST 1.1-defined tumour response (50 percent response rate, 7/12 partial responses, 2/12 complete responses). This appears to confirm that both necrosis and spores must be present in order to achieve a tumor response (the vadimezan+PR-104 therapy group had a 0% response rate).
Others research have seen a similar occurrence with a combination of Clostridium novyi-NT spores, the vascular disrupting agent Dolstatin-10 (D10), and the DNA crosslinking agent mitomycin C (MMC); in the absence of C. novyi-NT spores, appreciable tumour regressions were not observed with D10/MMC.
This idea of this team was supported by the use of an alternate vascular targeting agent to improve Clostridium spore colonization of rat rhabdomyosarcomas, particularly in smaller tumors. Our findings support the need of choosing necrotic tumors for CDEPT therapy in advance. Integrating noninvasive imaging techniques (such as MRI or CT) that are suitable technologies for predicting necrotic volume, followed by noninvasive detection of NmeNTR expression by PET to confirm tumor colonization by C. sporogenes, could provide a superior and reliable approach to patient selection that maximizes treatment benefits. For pre-clinical development and clinical-stage patient monitoring, non-invasive imaging of NmeNTR expression as a surrogate for vector distribution and spread will be critical. Currently, several fluorescent and near-infrared probes are used to monitor nitroreductase enzymatic activity in vitro and in vivo.
However, there are technological limits in terms of tissue penetration when employing these probes, as near-infrared fluorescent light is only expected to penetrate a few centimeters, making this approach inappropriate for normal clinical application. Because oxygen inhibits human one-electron nitroreduction but not bacterial two-electron nitroreduction, we previously hypothesized that bacterial NTRs could metabolize and trap PET imaging molecules now in clinical trials for non-invasive hypoxia detection. Unlike earlier hypoxia-based PET agents FMISO and HX4, flow cytometry is used to identify EF5 adducts using a commercial monoclonal antibody conjugated to a fluorophore. In vitro, NmeNTR showed activity with EF5, giving it an advantage over other enzyme/prodrug combinations.
The ability to forecast the level of therapeutic benefit by correlating the strength of the PET signal with known vector load and/or prodrug efficacy could be useful in the future. The feasibility of this method has recently been proven in proof-of-concept micro PET tests. NmeNTR's catalytic activity on a variety of anti-infective substrates was proved to be useful as an extra safety feature.
In theory, this will make the vector hypersensitive to substrates that can be utilized to safely eradicate the burden of infection at the conclusion of treatment or in the event of an adverse occurrence. This should help alleviate safety concerns about the use of foreign genes and bacterial delivery platforms, as well as speed up clinical development.
Although the antibiotic Metronidazole (FlagylTM) is known to be sensitive to members of the Clostridium genus, action with other anti-infective substrates may provide additional antibiotic resistance mitigation.
In conclusion, the research identified NmeNTR/PR-104 as a promising enzyme/prodrug combination for application in CDEPT and exhibited considerable anti-tumor activity in a mouse model. By manipulating the necrotic portion of tumors with a vascular disrupting chemical, we were able to demonstrate that necrosis is essential for successful germination of C. sporogenes spores (vadimezan).
NmeNTR's capacity to metabolize additional important substrates at the same time, such as the PET imaging agent EF5 and a variety of anti-infective drugs, gives it a particular edge over other enzyme/prodrug combinations now in preclinical research. This work is a critical first step toward nitroreductase-armed C. sporogenes entering clinical evaluation because it addresses some of the obstacles that are limiting clinical advancement of CDEPT.






_underway_during_sea_trials_on_11_May_2017.jpg)

0 Comments
Feedback will be Appreciated